Membrane Transport of Small
Molecules and the Electrical
Properties of Membranes
Molecules and the Electrical
Properties of Membranes
Because of its hydrophobic interior, the lipid bilayer of small cell membranes restricts
the passage of most polar molecules. leonardo bridge This barrier function allows the cell to maintain
concentrations of solutes in its cytosol that differ from those in the extracellular
fluid and in each of the intracellular membrane-enclosed compartments.
To benefit from this barrier, however, cells have had to evolve ways of transferring
specific water-soluble molecules and ions across their membranes in order to
ingest essential nutrients, excrete metabolic waste products, and regulate intracellular
ion concentrations. Cells use specialized membrane transport proteins to
accomplish this goal. The importance of such small molecule transport is reflected
in the large number of genes in all organisms that code for the transmembrane
transport proteins involved, which make up 15–30% of the membrane proteins in
all cells. Some mammalian cells, such as nerve and kidney cells, devote up to twothirds
of their total metabolic energy consumption to such transport processes.
Cells can also transfer macromolecules and even large particles across their
membranes, but the mechanisms involved in most of these cases differ from
those used for transferring small molecules, and they are discussed in Chapters
12 and 13.
We begin this chapter by describing some general principles of how small
water-soluble molecules traverse cell membranes. We then consider, in turn, the
two main classes of membrane proteins that mediate this transmembrane traffic:
transporters, which undergo sequential conformational changes to transport specific
small molecules across membranes, and channels, which form narrow pores,
allowing passive transmembrane movement, primarily of water and small inorganic
ions. Transporters can be coupled to a source of energy to catalyze active
transport, leonardo da vinci bridge design which together with selective passive permeability, creates large differences
in the composition of the cytosol compared with that of either the extracellular
fluid (Table 11–1) or the fluid within membrane-enclosed organelles. By
generating inorganic ion-concentration differences across the lipid bilayer, cell
membranes can store potential energy in the form of electrochemical gradients,
which drive various transport processes, convey electrical signals in electrically
excitable cells, and (in mitochondria, chloroplasts, and bacteria) make most of
the cell’s ATP. We focus our discussion mainly on transport across the plasma
membrane, but similar mechanisms operate across the other membranes of the
eukaryotic cell, as discussed in later chapters.
In the last part of the chapter, we concentrate mainly on the functions of ion
channels in neurons (nerve cells). In these cells, channel da vinci tank proteins perform at their
highest level of sophistication, enabling networks of neurons to carry out all the
astonishing feats your brain is capable of.
PRINCIPLES OF MEMBRANE TRANSPORT
We begin this section by describing the permeability properties of protein-free,
synthetic lipid bilayers. We then introduce some of the terms used to describe the
various forms of membrane transport blood; the resulting accumulation davinci catapult of cystine in the urine leads to the formation of
cystine stones in the kidneys.
All membrane transport proteins that have been studied in detail are multipass
transmembrane proteins—that is, their polypeptide chains traverse the lipid
bilayer multiple times. By forming a protein-lined pathway across the membrane,
these proteins enable specific hydrophilic solutes to cross the membrane without
coming into direct contact with the hydrophobic interior of the lipid bilayer.
Transporters and channels are the two major classes of membrane transport
proteins (Figure 11–3). Transporters (also called carriers, or permeases) bind the
specific solute to be transported and undergo a series of conformational changes
that alternately expose solute-binding sites on one side of the membrane and
then on the other to transfer the solute across it. Channels, by contrast, interact
with the solute to be transported much more weakly. They form continuous pores
that extend across the lipid bilayer. When open, these pores allow specific solutes
(such as inorganic ions of appropriate size and charge and in some cases small
molecules, including water, glycerol, and ammonia) to pass through them and
thereby cross the membrane. Leonardo da Vinci Inventions Not surprisingly, transport through channels occurs
at a much faster rate than transport mediated by transporters. Although water can
slowly diffuse across synthetic lipid bilayers, cells use dedicated channel proteins
(called water channels, or aquaporins) that greatly increase the permeability of
their membranes to water, as we discuss later.
Active Transport Is Mediated by Transporters Coupled to an
Energy Source
All channels and many transporters allow solutes to cross the membrane only
passively (“downhill”), a process leonardo da vinci helicopter called passive transport. In the case of transport
of a single uncharged molecule, the difference in the concentration on the two
sides of the membrane—its concentration gradient—drives passive transport and
determines its direction (Figure 11–4A). If the solute carries a net charge, however,
both its concentration gradient and the electrical potential difference across
the membrane, the membrane potential, influence its transport. da vinci tank The concentration
gradient and the electrical gradient combine to form a net driving force, the
electrochemical gradient, for each charged solute (Figure 11–4B). We discuss
electrochemical gradients in more detail later and in Chapter 14. In fact, almost all
plasma membranes have an electrical potential (i.e., a voltage) across them, with
the inside usually negative with respect to the outside. This potential favors the
entry of positively charged ions into the cell but opposes the entry of negatively
charged ions (see Figure 11–4B); it also opposes the efflux of positively charged
ions.
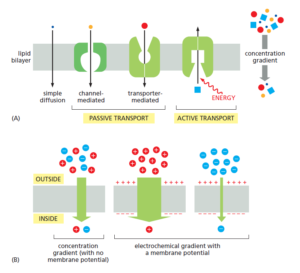
As shown in Figure 11–4A, in addition to passive transport, cells need to be
able to actively pump certain solutes across the membrane “uphill,” against their
electrochemical gradients. Such active transport is mediated by transporters
whose pumping activity is directional because it is tightly coupled to a source of
metabolic energy, such as an ion gradient or ATP self propelled car designs hydrolysis, as discussed later.
Transmembrane movement of small molecules mediated by transporters can be
either active or passive, whereas that mediated by channels is always passive
Summary
Lipid bilayers are virtually impermeable to most polar molecules. To transport
small water-soluble molecules into or out of cells or intracellular membrane-enclosed
compartments, cell membranes contain various membrane transport proteins,
each of which is responsible for transferring a particular solute or class of
solutes across the membrane. There are two classes of membrane transport proteins—
transporters and channels. Both form protein pathways across the lipid
bilayer. Whereas transmembrane movement mediated by transporters can be
either active or passive, solute flow through channel proteins is always passive. Both
active and passive ion transport is influenced by the ion’s concentration gradient
and the membrane potential—that is, its electrochemical gradient.
Intracellular Compartments
and Protein Sorting
Unlike a bacterium, which generally consists of a single intracellular compartment
surrounded by a plasma membrane, a eukaryotic cell is elaborately subdivided
into functionally distinct, membrane-enclosed compartments. Each
compartment, or organelle, contains its own characteristic set of enzymes and
other specialized molecules, and complex distribution systems transport specific
products from one compartment to another. To understand the eukaryotic cell, it
is essential to know how the cell creates and maintains these compartments, what
occurs in each of them, and how molecules move between them.
Proteins confer upon each compartment its characteristic structural and
functional properties. They catalyze the reactions that occur there and selectively
transport small molecules into and out of the compartment. For membrane-enclosed
organelles in the cytoplasm, proteins also serve as organelle-specific surface
markers that direct new deliveries of proteins and lipids to the appropriate
organelle.
An animal cell contains about 10 billion (1010) protein molecules of perhaps
10,000 kinds, and the synthesis of almost all of them begins in the cytosol, the
space of the cytoplasm outside the membrane-enclosed organelles. Each newly
synthesized protein is then delivered specifically to the organelle that requires it.
The intracellular transport of proteins is the central theme of both this chapter
and the next. By tracing the protein traffic from one compartment to another, one
can begin to make sense of the otherwise bewildering maze of intracellular membranes.
The Compartmentalization of Cells
In this brief overview of the compartments of the cell and the relationships
between them, we organize the organelles conceptually into a small number of
discrete families, discuss how proteins are directed to specific organelles, and
explain how proteins cross organelle membranes. leonardo da vinci self supporting bridge
All Eukaryotic Cells Have the Same Basic Set of Membraneenclosed
Organelles
Many vital biochemical processes take place in membranes or on their surfaces.
Membrane-bound enzymes, for example, catalyze lipid metabolism; and oxidative
phosphorylation and photosynthesis both require a membrane to couple the
transport of H+ to the synthesis of ATP. In addition to providing increased membrane
area to host biochemical reactions, intracellular membrane systems form
enclosed compartments that are separate from the cytosol, thus creating functionally
specialized aqueous spaces within the cell. In these spaces, subsets of molecules
(proteins, reactants, ions) are concentrated to optimize the biochemical
reactions in which they participate. Because the lipid bilayer of cell membranes is
impermeable to most hydrophilic molecules, the membrane of an organelle must
contain membrane transport proteins to import and export specific metabolites.
Each organelle membrane must also have a mechanism for importing, and incorporating
into the organelle, the specific proteins that make the organelle unique.
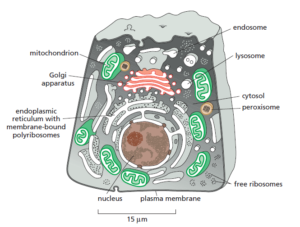
About half the total area of membrane in a eukaryotic cell encloses the labyrinthine
spaces of the endoplasmic reticulum (ER). The rough ER has many ribosomes
bound to its cytosolic surface. Ribosomes are organelles that are not membrane-
enclosed; they synthesize both soluble and integral membrane proteins,
most of which are destined either for secretion to the cell exterior or for other
organelles. We shall see that, whereas proteins are transported into other membrane-
enclosed organelles only after their synthesis is complete, they are transported
into the ER as they are synthesized. This explains why the da vinci helicopter ER membrane is
unique in having ribosomes tethered to it. The ER also produces most of the lipid
for the rest of the cell and functions as a store for Ca2+ ions. Regions of the ER that
lack bound ribosomes are called smooth ER. The ER sends many of its proteins
and lipids to the Golgi apparatus, which often consists of organized stacks of disclike
compartments called Golgi cisternae. The Golgi apparatus receives lipids and
proteins from the ER and dispatches them to various destinations, usually covalently
modifying them en route.
Mitochondria and chloroplasts generate most of the ATP that cells use to drive
reactions requiring an input of free energy; chloroplasts are a specialized version
of plastids (present in plants, algae, and some protozoa), which can also have
other functions, such as the storage of food or pigment molecules. Lysosomes contain
digestive enzymes that degrade defunct intracellular organelles, as well as
macromolecules and particles taken in from outside the cell by endocytosis. On
the way to lysosomes, endocytosed material must first pass through a series of
organelles called endosomes. Finally, peroxisomes are small vesicular compartments
that contain enzymes used in various oxidative reactions.
In general, each membrane-enclosed organelle performs the same set of basic
functions in all cell types. But to serve the specialized functions of cells, these
organelles vary in abundance and can have additional properties that differ from
cell type to cell type.
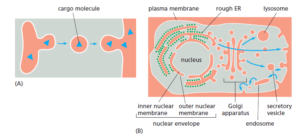
To understand the general principles by which sorting signals operate, it is
important to distinguish three fundamentally different ways by which proteins
move from one compartment to another. These three mechanisms are described
below, and the transport steps at which they operate are outlined in Figure 12–5.
We discuss the first two mechanisms (gated transport and transmembrane transport)
in this chapter, and the third (vesicular transport, green arrows in Figure
12–5) in Chapter 13.
1. In gated transport, proteins and RNA molecules move between the cytosol
and the nucleus through nuclear pore complexes in the nuclear envelope.
The nuclear pore complexes function as selective gates that support the
active transport of specific macromolecules davinci catapult and macromolecular assemblies
between the two topologically equivalent spaces, although they also
allow free diffusion of smaller molecules.
2. In protein translocation, transmembrane protein translocators directly
transport specific proteins across a membrane from the cytosol into a
space that is topologically distinct. The transported protein molecule usually
must unfold to snake through the translocator. The initial transport
of selected proteins from the cytosol into the ER lumen or mitochondria,
for example, occurs in this way. Integral membrane proteins often use the
same translocators but translocate only partially across the membrane, so
that the protein becomes embedded in the lipid bilayer.
3. In vesicular transport, membrane-enclosed transport intermediates—
which may be small, spherical transport vesicles or larger, irregularly
shaped organelle fragments—ferry proteins from one topologically equivalent
compartment to another. The transport vesicles and fragments become
loaded with a cargo of molecules derived from the lumen of one compartment
as they bud and pinch off from its membrane; they discharge their
cargo into a second compartment by fusing with the membrane enclosing
that compartment (Figure 12–6). The transfer of soluble leonardo da vinci tank proteins from
the ER to the Golgi apparatus, for example, occurs in this way.
Membrane Transport of Small
Molecules and the Electrical
Properties of Membranes
Because of its hydrophobic interior, the lipid bilayer of cell membranes restricts
the passage of most polar molecules. This barrier function allows the cell to maintain
concentrations of solutes in its cytosol that differ from those in the extracellular
fluid and in each of the intracellular membrane-enclosed compartments.
To benefit from this barrier, however, cells have had to evolve ways of transferring
specific water-soluble molecules da vinci bridge design and ions across their membranes in order to
ingest essential nutrients, excrete metabolic waste products, and regulate intracellular
ion concentrations. Cells use specialized membrane transport proteins to
accomplish this goal. The importance of such small molecule transport is reflected
in the large number of genes in all organisms that code for the transmembrane
transport proteins involved, which make up 15–30% of the membrane proteins in
all cells. Some mammalian cells, such as nerve and kidney cells, devote up to twothirds
of their total metabolic energy consumption to such transport processes.
Cells can also transfer macromolecules and even large particles across their
membranes, but the mechanisms involved in most of these cases differ from
those used for transferring small molecules, and they are discussed in Chapters
12 and 13.
We begin this chapter by describing some general principles of how small
water-soluble molecules traverse cell membranes. We then consider, in turn, the
two main classes of membrane proteins that mediate this transmembrane traffic:
transporters, which undergo sequential conformational changes to transport specific
small molecules across membranes, and channels, which form narrow pores,
allowing passive transmembrane movement, primarily of water and small inorganic
ions. Transporters can be coupled to a source of energy to catalyze active
transport, which together with selective passive permeability, creates large differences
in the composition of the cytosol compared with that of either the extracellular
fluid (Table 11–1) or the fluid within membrane-enclosed organelles. By
generating inorganic ion-concentration differences across the lipid bilayer, cell
membranes can store potential energy in the form of electrochemical gradients,
which drive various transport processes, convey electrical signals in electrically
excitable cells, and (in mitochondria, chloroplasts, and bacteria) make most of
the cell’s ATP. We focus our discussion mainly on transport across the plasma
membrane, but similar mechanisms operate across the other membranes of the
eukaryotic cell, as discussed in later chapters.
In the last part of the chapter, we concentrate mainly on the functions of ion
channels in neurons (nerve cells). In these cells, channel da vinci tank proteins perform at their
highest level of sophistication, enabling networks of neurons to carry out all the
astonishing feats your brain is capable of.
PRINCIPLES OF MEMBRANE TRANSPORT
We begin this section by describing the permeability properties of protein-free,
synthetic lipid bilayers. We then introduce some of the terms used to describe the
various forms of membrane transport blood; the resulting accumulation davinci catapult of cystine in the urine leads to the formation of
cystine stones in the kidneys.
All membrane transport proteins that have been studied in detail are multipass
transmembrane proteins—that is, their polypeptide chains traverse the lipid
bilayer multiple times. By forming a protein-lined pathway across the membrane,
these proteins enable specific hydrophilic solutes to cross the membrane without
coming into direct contact with the hydrophobic interior of the lipid bilayer.
Transporters and channels are the two major classes of membrane transport
proteins (Figure 11–3). Transporters (also called carriers, or permeases) bind the
specific solute to be transported and undergo a series of conformational changes
that alternately expose solute-binding sites on one side of the membrane and
then on the other to transfer the solute across it. Channels, by contrast, interact
with the solute to be transported much more weakly. They form continuous pores
that extend across the lipid bilayer. When open, these pores allow specific solutes
(such as inorganic ions of appropriate size and charge and in some cases small
molecules, including water, glycerol, and ammonia) to pass through them and
thereby cross the membrane. Leonardo da Vinci Inventions Not surprisingly, transport through channels occurs
at a much faster rate than transport mediated by transporters. Although water can
slowly diffuse across synthetic lipid bilayers, cells use dedicated channel proteins
(called water channels, or aquaporins) that greatly increase the permeability of
their membranes to water, as we discuss later.
Active Transport Is Mediated by Transporters Coupled to an
Energy Source
All channels and many transporters allow solutes to cross the membrane only
passively (“downhill”), a process leonardo da vinci helicopter called passive transport. In the case of transport
of a single uncharged molecule, the difference in the concentration on the two
sides of the membrane—its concentration gradient—drives passive transport and
determines its direction (Figure 11–4A). If the solute carries a net charge, however,
both its concentration gradient and the electrical potential difference across
the membrane, the membrane potential, influence its transport. da vinci tank The concentration
gradient and the electrical gradient combine to form a net driving force, the
electrochemical gradient, for each charged solute (Figure 11–4B). We discuss
electrochemical gradients in more detail later and in Chapter 14. In fact, almost all
plasma membranes have an electrical potential (i.e., a voltage) across them, with
the inside usually negative with respect to the outside. This potential favors the
entry of positively charged ions into the cell but opposes the entry of negatively
charged ions (see Figure 11–4B); it also opposes the efflux of positively charged
ions.

Membrane transport
As shown in Figure 11–4A, in addition to passive transport, cells need to be
able to actively pump certain solutes across the membrane “uphill,” against their
electrochemical gradients. Such active transport is mediated by transporters
whose pumping activity is directional because it is tightly coupled to a source of
metabolic energy, such as an ion gradient or ATP self propelled car designs hydrolysis, as discussed later.
Transmembrane movement of small molecules mediated by transporters can be
either active or passive, whereas that mediated by channels is always passive
Summary
Lipid bilayers are virtually impermeable to most polar molecules. To transport
small water-soluble molecules into or out of cells or intracellular membrane-enclosed
compartments, cell membranes contain various membrane transport proteins,
each of which is responsible for transferring a particular solute or class of
solutes across the membrane. There are two classes of membrane transport proteins—
transporters and channels. Both form protein pathways across the lipid
bilayer. Whereas transmembrane movement mediated by transporters can be
either active or passive, solute flow through channel proteins is always passive. Both
active and passive ion transport is influenced by the ion’s concentration gradient
and the membrane potential—that is, its electrochemical gradient.
Intracellular Compartments
and Protein Sorting
Unlike a bacterium, which generally consists of a single intracellular compartment
surrounded by a plasma membrane, a eukaryotic cell is elaborately subdivided
into functionally distinct, membrane-enclosed compartments. Each
compartment, or organelle, contains its own characteristic set of enzymes and
other specialized molecules, and complex distribution systems transport specific
products from one compartment to another. To understand the eukaryotic cell, it
is essential to know how the cell creates and maintains these compartments, what
occurs in each of them, and how molecules move between them.
Proteins confer upon each compartment its characteristic structural and
functional properties. They catalyze the reactions that occur there and selectively
transport small molecules into and out of the compartment. For membrane-enclosed
organelles in the cytoplasm, proteins also serve as organelle-specific surface
markers that direct new deliveries of proteins and lipids to the appropriate
organelle.
An animal cell contains da Vinci bridge about 10 billion (1010) protein molecules of perhaps
10,000 kinds, and the synthesis of almost all of them begins in the cytosol, the
space of the cytoplasm outside the membrane-enclosed organelles. Each newly
synthesized protein is then delivered specifically to the organelle that requires it.
The intracellular transport of proteins is the central theme of both this chapter
and the next. By tracing the protein traffic from one compartment to another, one
can begin to make sense of the otherwise bewildering maze of intracellular membranes.
The Compartmentalization of Cells
In this brief overview of the compartments of the cell and the relationships
between them, we organize the organelles conceptually into a small number of
discrete families, discuss how proteins are directed to specific organelles, and
explain how proteins cross organelle membranes.
All Eukaryotic Cells Have the Same Basic Set of Membraneenclosed
Organelles
Many vital biochemical processes take place in membranes or on their surfaces.
Membrane-bound enzymes, for example, catalyze lipid metabolism; and oxidative
phosphorylation and photosynthesis both require a membrane to couple the
transport of H+ to the synthesis of ATP. In addition to providing increased membrane
area to host biochemical leonardo da vinci bridge reactions, intracellular membrane systems form
enclosed compartments that are separate from the cytosol, thus creating functionally
specialized aqueous spaces within the cell. In these spaces, subsets of molecules
(proteins, reactants, ions) are concentrated to optimize the biochemical
reactions in which they participate. Because the lipid bilayer of cell membranes is
impermeable to most hydrophilic molecules, the membrane of an organelle must
contain membrane transport proteins to import and export specific metabolites.
Each organelle membrane must also have a mechanism for importing, and incorporating
into the organelle, the specific proteins that make the organelle unique.

About half the total area of membrane in a eukaryotic cell encloses the labyrinthine
spaces of the endoplasmic reticulum (ER). The rough ER has many ribosomes
bound to its cytosolic surface. Ribosomes are organelles that are not membrane-
enclosed; they synthesize both soluble and integral membrane proteins,
most of which are destined either for secretion to the cell exterior or for other
organelles. We shall see that, whereas proteins are transported into other membrane-
enclosed organelles only after their synthesis is complete, they are transported
into the ER as they are synthesized. This explains why the da vinci helicopter ER membrane is
unique in having ribosomes tethered to it. The ER also produces most of the lipid
for the rest of the cell and functions as a store for Ca2+ ions. Regions of the ER that
lack bound ribosomes are called smooth ER. The ER sends many of its proteins
and lipids to the Golgi apparatus, which often consists of organized stacks of disclike
compartments called Golgi cisternae. The Golgi apparatus receives lipids and
proteins from the ER and dispatches them to various destinations, usually covalently
modifying them en route.
Mitochondria and chloroplasts generate most of the ATP that cells use to drive
reactions requiring an input of free energy; chloroplasts are a specialized version
of plastids (present in plants, algae, and some protozoa), which can also have
other functions, such as the storage of food or pigment molecules. Lysosomes contain
digestive enzymes that degrade defunct intracellular organelles, as well as
macromolecules and particles taken in from outside the cell by endocytosis. On
the way to lysosomes, endocytosed material must first pass through a series of
organelles called endosomes. Finally, peroxisomes are small vesicular compartments
that contain enzymes used in various oxidative reactions.
In general, each membrane-enclosed organelle performs the same set of basic
functions in all cell types. But to serve the specialized functions of cells, these
organelles vary in abundance and can have additional properties that differ from
cell type to cell type.

To understand the general principles by which sorting signals operate, it is
important to distinguish three fundamentally different ways by which proteins
move from one compartment to another. These three mechanisms are described
below, and the transport steps at which they operate are outlined in Figure 12–5.
We discuss the first two mechanisms (gated transport and transmembrane transport)
in this chapter, and the third (vesicular transport, green arrows in Figure
12–5) in Chapter 13.
1. In gated transport, proteins and RNA molecules move between the cytosol
and the nucleus through nuclear pore complexes in the nuclear envelope.
The nuclear pore complexes function as selective gates that support the
active transport of specific macromolecules davinci catapult and macromolecular assemblies
between the two topologically equivalent spaces, although they also
allow free diffusion of smaller molecules.
2. In protein translocation, transmembrane protein translocators directly
transport specific proteins across a membrane from the cytosol into a
space that is topologically distinct. The transported protein molecule usually
must unfold to snake through the translocator. The initial transport
of selected proteins from the cytosol into the ER lumen or mitochondria,
for example, occurs in this way. Integral membrane proteins often use the
same translocators but translocate only partially across the membrane, so
that the protein becomes embedded in the lipid bilayer.
3. In vesicular transport, membrane-enclosed transport intermediates—
which may be small, spherical transport vesicles or larger, irregularly
shaped organelle fragments—ferry proteins from one topologically equivalent
compartment to another. The transport vesicles and fragments become
loaded with a cargo of molecules derived from the lumen of one compartment
as they bud and pinch off from its membrane; they discharge their
cargo into a second compartment by fusing with the membrane enclosing
that compartment (Figure 12–6). The transfer of soluble leonardo da vinci tank proteins from
the ER to the Golgi apparatus, for example, occurs in this way.